
Product
NeoMetrix Dx, Inc. is developing the world’s first automated in-vitro medical diagnostic device (“AL4 Dx”) that tests for both total bilirubin (BT) and unbound bilirubin (Bf). In addition, a patented proprietary algorithm will offer clinicians insight into the newborn’s unique bilirubin binding characteristics. This novel comprehensive panel will provide clinicians with personalized information to accurately diagnose neonatal risk of Hyperbilirubinemia in an efficient, and cost-effective manner.
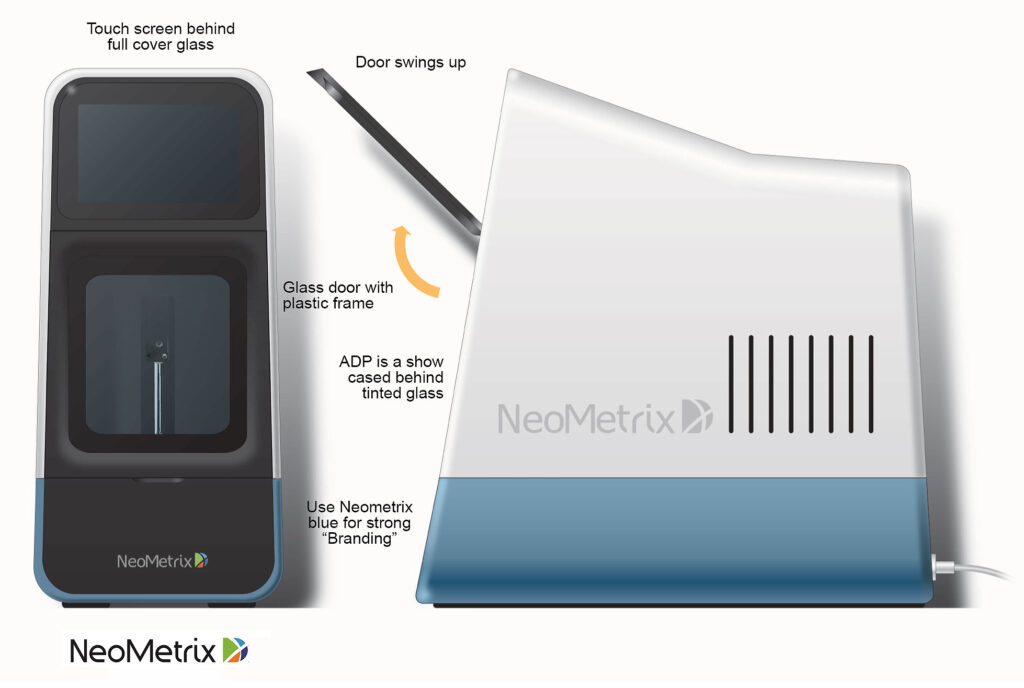
AL4 Dx is a computerized, compact in-vitro diagnostic instrument built around a robotic pipetting system and multi wavelength spectrophotometer.
- AL4 Dx automates the complex wet chemical procedures to measure both Bf and BT using microliter blood samples appropriate to newborns.
- AL4 Dx further calculates the newborn’s unique bilirubin binding variables BTmax and KA using a proprietary algorithm that will help clinicians to determine personalized treatment plans based upon each newborn’s unique binding characteristics